All News
Cummings Awarded NIH Grant to Inform Malaria Vaccine Design
Despite decades of eradication efforts, malaria remains a global cause of disease and death, with the greatest burden largely affecting young children in Sub-Saharan Africa.
In 2021, an estimated 247 million people worldwide were infected with malaria resulting in 619,000 deaths, according to the World Health Organization (WHO).
That same year, the WHO approved the first malaria vaccine which has shown to significantly reduce deaths among young children. However, compared to other adolescent vaccinations, it has modest efficacy, preventing only about 30 percent of severe malaria cases.
Supported by a $483,000 grant from the National Institutes of Health (NIH), Michael Cummings, a professor of biology with an appointment in the University of Maryland Institute for Advanced Computer Studies, is working to understand the immune response to malaria to help scientists develop more effective vaccines.
Along with Cummings, who brings extensive biological data science expertise, the research team is comprised of Assistant Professor Andrea Berry, a pediatric infectious disease physician and malaria expert at the University of Maryland School of Medicine, and Assistant Professor John Altin, an immunology and genomics expert at the Translational Genomics Research Institute.
Their research investigates immune responses to malaria infection in infants and children in the Sub-Saharan countries Mali and Malawi, which are geographically distant sites with different malaria transmission patterns.
Cummings, who is also the director of the Center for Bioinformatics and Computational Biology, says the bulk of the data will be collected using a new customizable lab platform called PepSeq. The platform, which Altin helped create, starts with designing a “library” of peptides of interest—short strings of amino acids that are the building blocks of proteins. Each peptide is then linked to a unique DNA tag, which allows scientists to pinpoint which peptides are being targeted by which antibodies (or other proteins) in a sample.
The team will use a combination of statistical and machine learning approaches to analyze hundreds of thousands of peptides, with the goal of identifying antigens that will help inform vaccine development.
—Story by Melissa Brachfeld, UMIACS communications group
UMD Researchers Partner with Wilmer Eye Institute to Enhance Sustained Delivery of Ocular Drugs
Computational biologists at the University of Maryland are partnering with ophthalmologists from the renowned Wilmer Eye Institute at John Hopkins University to improve therapeutic options for people suffering from chronic ocular diseases.
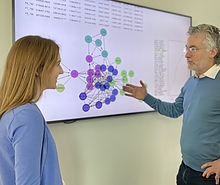
Michael Cummings (left in photo) a professor of biology with an appointment in the University of Maryland Institute for Advanced Computer Studies, and Renee Ti Chou (right), a fourth-year computational biology doctoral student, are using machine learning algorithms and other methods to enhance how certain drugs are administered to patients suffering from serious eye ailments.
Their research is covered in-depth in a paper published on May 2, 2023, in the prestigious journal Nature Communications.
According to the Centers for Disease Control and Prevention, more than 4.2 million Americans aged 40 years and older are either legally blind or have low vision, primarily due to age-related eye diseases such as macular degeneration, cataracts, diabetic retinopathy, and glaucoma.
Surgery is an option for some, but for many others, treatment involves strictly adhering to eyedrop dosing regimens or frequent intraocular injections. This has proven to be problematic, with studies showing that only 64% of patients being treated for glaucoma adhere to the three-times daily eyedrop dosing schedule over a four-week period.
Implantable drug delivery systems remain a promising option for the sustained, controlled delivery of therapeutics in the eye, but they come with their own set of risks, including adverse reactions to the cornea and other side effects.
The UMD researchers, working with ophthalmologists, bioengineers, and pharmacologists at The John Hopkins University School of Medicine—home to the Wilmer Eye Institute—have an alternative solution, one that involves a process known as peptide engineering.
Chou, who is the co-lead author of the paper, says the key to their work is to improve the drug binding properties in ocular melanin, which is found in pigmented eye tissues and protects the eye from harmful ultraviolent light. Ocular melanin can act as a sustained-release depot in the eye for drug therapeutics, Chou adds, allowing the drugs to remain active for longer periods, which translates to less eyedrops or intraocular injections.
But not all drugs bind well to melanin, so the researchers are hoping to use peptides, which are short sequences of amino acids, as an “adaptor” to impart the melanin binding property to non-melanin binding drugs.
“In our study, we linked the candidate peptide to an ocular drug through conjugation and demonstrated that it can increase the drug retention time from eight days to up to 18 days, using an intraocular pressure reducing drug called brimonidine as an example,” Chou says.
Cummings, who is director of the Center for Bioinformatics and Computational Biology, says they used machine learning-based analytical methodology to develop the peptide adaptor, which is able to engineer new types of peptides that have a high cell-penetrating ability coupled with low cytotoxicity properties.
“We believe this method will have a distinct advantage over current implantable devices, as it does not require surgery or device removal, and is expected to have fewer side effects, both of which can improve a patient’s quality of life,” says Chou.
—Story by Melissa Brachfeld, UMIACS communications group
CBCB Researchers Present Four Papers at Top Conference on Computational Molecular Biology

Researchers in the Center for Bioinformatics and Computational Biology (CBCB) have four papers accepted to the Conference on Research in Computational Molecular Biology (RECOMB 2023), held this year from April 16–19 in Istanbul.
The papers—which introduce new methods and tools to improve genome sequencing so that scientists can better study evolutionary trees, tumors and cancer—were coauthored by Erin Molloy, an assistant professor of computer science; Rob Patro, an associate professor of computer science; and their graduate students.
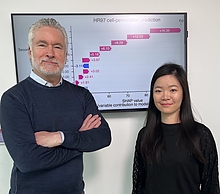
“RECOMB is long established as one of the very best international conferences in computational biology and bioinformatics,” says Michael Cummings, a professor of biology and director of CBCB. “The fact that collectively Rob and Erin, together with their students and collaborators, have four papers accepted, is demonstrative of the quality of their research. We are very pleased with their success.”
As part of CBCB, Cummings, Molloy (pictured center), and Patro have dual appointments in the University of Maryland Institute for Advanced Computer Studies.
One of Molloy’s papers, “TREE-QMC: Improving quartet graph construction for scalable and accurate species tree estimation from gene trees,” introduces a new method for estimating evolutionary trees.
Reconstructing the evolutionary history of life on Earth is a scientific grand challenge. Parts of this history have proven difficult to resolve because of highly heterogeneous and error-prone input data, she says.
In their paper, Molloy and Yunheng Han, a fourth-year computer science doctoral student, revisit a graph-based approach for building evoluntionary trees. Han and Molloy introduce efficient algorithms for graph construction and normalization, enabling their new method to be faster and more robust to error and heterogeneity than state-of-the-art methods.
The second paper being presented, “Spectrum preserving tilings enable sparse and modular reference indexing,” focuses on finding ways to efficiently index genomic sequences.
Many DNA and RNA sequence analyses query short sequences of fixed length, termed k-mers, against a collection of known reference sequences, like in human or bacterial genomes. To do so, analyses and algorithms use an index to rapidly find where each k-mer occurs on which reference.
In their paper, the researchers show how a class of these indexes can be broken down into two modular pieces, and how these modular indexes can be made both fast and small. 
“If one were to modularly compose a state-of-the-art hash function with our compression algorithm, an index built over 30,000 bacterial genomes could be $300 cheaper per month to host on Amazon Web Services,” he explains.
Fan’s coauthors include Patro (pictured left); Jamshed Khan, a fourth-year computer science doctoral student; and Giulio Ermanno Pibiri, an assistant professor of computer science at Ca’ Foscari University of Venice.
Yuelin Liu, a fourth-year computer science doctoral student and visiting fellow at the National Cancer Institute (NCI), is the lead author of a paper that introduces a new tool to reconstruct tumor lineage trees so that scientists can better understand how tumors evolve, potentially leading to more effective and targeted treatments.
In addition to her coauthors at NCI, Liu’s UMD coauthors include Molloy; Xuan Cindy Li, a doctoral student of biological science who is also a visiting fellow at NCI; David R. Crawford, another biological science doctoral student and research fellow at NCI; Stephen Mount, an associate professor of cell biology and molecular genetics; and Eytan Ruppin, formerly a professor of computer science and director of CBCB who is now chief of NCI’s Cancer Data Science Laboratory.
The evolution of a tumor can be modeled as a phylogenetic tree, and the goal of single-cell tumor lineage reconstruction is to trace such history using single-cell sequencing data from the lesions of a cancer patient, says Liu. However, constructing tumor lineage trees with a single-cell mutation or copy number data often suffers from data sparsity.
In their paper, the researchers introduce Sgootr, a tool to jointly infer a tumor lineage tree and identify lineage-informative CpG sites from single-cell methylation sequencing data.
Methylation is a biological process by which methyl groups are added to the DNA molecule and change the activity of a DNA segment without changing the sequence. CpG methylation is widely studied type of epigenetic modification.
Using their tool Sgootr, the team successfully reconstructed tumor lineage trees from both real and simulated single-cell methylation datasets, showing that CpG methylation harbors rich signals to evaluate tumors.
The final paper, “TreeTerminus–Creating transcript trees using inferential replicate counts,” was accepted to RECOMB-Seq, a satellite conference that took place April 14–15. It was coauthored by Noor Pratap Singh, a fourth-year computer science doctoral student; Michael I. Love, an associate professor of biostatistics and genetics at the University of North Carolina at Chapel Hill; and Patro.
Genes and transcripts are the two most common base units of analysis for an RNA-sequencing dataset. While transcripts provide the finest resolution of analysis, it might be hard to infer the true abundance estimates for some of them. On the other hand, the gene abundance estimates are more accurate, but there is also a risk of losing the underlying information of which transcripts are responsible for the observed effects.
“Our method tries to get the best of both worlds, by arranging transcripts in a tree-like structure, where we get more confident about the abundance estimates as we climb up the tree,” Singh says. “We then propose a method to find the appropriate nodes from the tree and use those nodes as the base unit of downstream analysis. The nodes represent aggregated transcript sets and are quite often at a level below genes with a more certain abundance estimate.”
The paper by Singh, Love and Patro was recognized with a Best Paper/Talk award at the satellite conference.
—Story by Melissa Brachfeld, UMIACS communications group
Cummings Awarded Grand Challenges Grant to Advance Genetic Analyses
Michael Cummings, a professor of biology with an appointment in the University of Maryland Institute for Advanced Computer Studies, is the recipient of a $50,000 Individual Project Grant that will support his work in analyzing genetic ancestry more accurately at the sub-chromosome level.
The award comes from the university’s Grand Challenges Grants Program, a $30 million initiative to support research that offers promising solutions to emerging societal issues like climate change, social injustice, global health and education disparities.
Cummings’ three-year project, "Accurate, Equitable and Transparent Genetic Ancestry Inference," embodies social justice, health and fair and trustworthy technology.
"To fully achieve healthcare justice and separate genetic factors from social and environmental sources of health disparity, we need to recognize the genetic diversity of individuals at the sub-chromosome level," explains Cummings, who is the director of the Center for Bioinformatics and Computational Biology.
Collaborating with Cummings is his advisee Alexis Boleda, a fourth-year Ph.D. student in the Biological Sciences Graduate Program.
Boleda works with Cummings to develop and evaluate new algorithms and data structures for the project.
"We already have a framework for how the method works," she says. "Now we are testing different alternatives to increase accuracy and computational efficiency."
Cummings and Boleda hope their research will provide a more accurate and comprehensive method for separating genetic factors from social and environmental sources of health disparity.
On a broader level, their work can help scientists better understand the principles behind population genetics, evolution, and the genetic basis of human biology.
–Story by Ethan Cannistra, UMIACS communications group
$3M NIH Grant Supports Genomic Approach to Curing ‘Neglected’ Disease

Supported by a new $3 million grant, a University of Maryland genomicist is continuing a decadelong fight against a painful, disfiguring parasitic skin condition that has received scant attention from the global medical community because of who it infects—the world’s poorest people.
The funding from the National Institute of Allergy and Infectious Diseases (NIAID) allows Professor of Cell Biology & Molecular Genetics Najib El-Sayed and partners in South America to use powerful sequencing tools to combat the Leishmania parasite, which is transmitted through sandfly bites and causes the disease cutaneous leishmaniasis.
The World Health Organization estimates up to 1 million new cases arise each year, mostly in tropical or sub-tropical North Africa and South America—regions already burdened with malnutrition, population displacement, poor housing and scarce financial resources.
One of the most daunting aspects of the disease is the parasite’s ability to defy most treatment options, either by resisting antiprotozoal drugs altogether or going dormant, only to reemerge years later. El-Sayed’s team will collaborate with Maria Adelaida Gómez, a microbiologist at the International Center for Medical Training and Research (CIDEIM) in Cali, Colombia, to address these “persister” leishmaniasis infections.
They’ll do this by working to identify microenvironmental conditions in human skin and organs that encourage and harbor persister populations of the Leishmania parasite, leading to better diagnostic and therapeutic outcomes to treat this devasting disease.
Colombia has the second-highest number of cases of cutaneous leishmaniasis in South America, with more than 100,000 infections reported in the past decade, the researchers said. Many of those affected are women and children, and the ulcers and scars from the disease can lead to psychological trauma and social stigma, severely impacting children’s development and adults’ ability to earn a living.
“My greatest interest in the exposure [of our research] are the folks impacted by this and other neglected diseases of poverty,” El-Sayed said. “The work we're doing with Maria Adelaida Gómez has moved us from basic research to the translational realm, with direct impact.”
The skin form of the disease is the most common. Other variants include mucocutaneous leishmaniasis, affecting the mouth, nose and throat, and visceral leishmaniasis, which attacks the spleen and liver and is almost always fatal without treatment.
No vaccine can counter Leishmania, Gómez said, and the drugs currently used to treat the disease are frequently ineffective, toxic and difficult to administer to those who need them most.
“Imagine yourself in the middle of the jungle, where you must travel five hours by boat or by foot to the closest village,” she said. “That’s the kind of trip an average patient in Colombia has to do every day for 20 days, just to receive a toxic treatment.”
El-Sayed and Gómez had previously collaborated on a $2.5 million NIAID grant focused on what the body’s immune response and inflammation indicates about the clinical outcome of anti-leishmanial drug treatments.
That earlier work focused on how Leishmania parasites interact with macrophages, a type of white blood cell in humans that circulate the body in search of unnecessary or harmful substances to destroy. Macrophages are key to our immune response, El-Sayed said, because they can recognize pathogens, capture them and destroy them. But Leishmania is unique in that it can enter macrophage cells and live within them.
“It’s an incredible parasite—it’s attacking the very central cell of your immune system,” said El-Sayed, who has an appointment in the University of Maryland Institute for Advanced Computer Studies (UMIACS).
Using computational power provided by UMIACS, the Maryland researchers will access the large volume of data that both teams have collected, running new algorithms that can better determine how and why persister parasites turn “on” and “off.”
Ultimately, this approach could shift the focus for treating Leishmania infections on a broad scale—from trying to kill the parasite with drugs of questionable effectiveness and safety to one seeking to ensure it never flips the “on” switch in the body of an infected person.
“I envision that results from this new study will unravel the mechanisms used by parasites to survive within their host without causing disease,” Gómez said. “This will bring us closer to understanding how protozoan parasites have co-evolved with their hosts. It will also allow us to develop new treatment methods that are not as toxic, shifting the paradigm of anti-leishmanial therapeutics from killing the bug by intoxicating the host, to promoting ‘healthy’ host-pathogen relationships.”
—Story by Melissa Brachfeld, UMIACS communications group
21 UMIACS Faculty Involved in Eight Grand Challenge Grants

The University of Maryland has announced an investment of more than $30 million in funding to address major societal issues ranging from climate change to illiteracy to pandemic preparedness and much more.
The Grand Challenges Grants program will support 50 projects from across the campus, with 21 faculty from the University of Maryland Institute for Advanced Computer Studies (UMIACS) involved in eight of them.
“This is fantastic news that truly represents the breadth and impact of our research enterprise,” says Mihai Pop, a professor of computer science and the director of UMIACS. “The significant number of faculty involved—representing seven academic departments—speaks directly to our mission of using interdisciplinary research and scholarship to address grand challenges in science and society. I couldn't be prouder of my colleagues.”
See below for a summary of projects UMIACS faculty are involved in.
• Of the six impact grants awarded, UMIACS faculty are active in three: The “Values-Centered AI Initiative” project includes Hal Daumé, Katie Shilton, John Horty, Jordan Boyd-Graber, Furong Huang, Vanessa Frias-Martinez, Louiqa Raschid, Marine Carpuat, Pratap Tokekar, John Dickerson and Huaishu Peng; the “Center of Excellence in Microbiome Sciences” includes Brantley Hall and Mihai Pop; and the “Maryland Institute for Digital Accessibility” includes Huaishu Peng, Niklas Elmqvist, Ge Gao and Abhinav Shrivastava.
• Of the 16 team project grants awarded, UMIACS is active in four: The “Music Education for All” project includes Cornelia Fermüller; the “Fostering Inclusivity Through Technology” project includes Louiqa Raschid, Niklas Elmqvist and Ge Gao; Christopher Metzler and Maria Molina are co-PIs for their “Effective and Equitable Weather Forecasting in a Changing Climate” project; and Wei Ai is part of the “M-Powering Teachers: ML to Improve Equity in K–12 Math” project.
• Michael Cummings was the recipient of a single PI project grant for his work involving “Accurate, Equitable, and Transparent Genetic Ancestry Inference.”
—Article by UMIACS communications group
CBCB Doctoral Student Takes Genomics Research to New Heights

Whether it’s scrambling up sheer rock faces, trying to solve a complex problem in the lab, or working long hours to submit an academic paper, Jason Fan is always eager to take on the next challenge.
Fan is a fifth-year computer science doctoral student in the Center for Bioinformatics and Computational Biology (CBCB) focused on designing small, fast, and efficient algorithms and tools for analyzing DNA and RNA sequencing data.
He’s also an avid rock climber, having participated in collegiate sport and speed competitions as an undergraduate at Tufts University, and nowadays—when time and weather permit—visiting the New River Gorge National Park and Preserve in West Virginia.
Fan says his academic life and passion for rock climbing go hand in hand.
“I fell in love with the problem solving and meditative aspects of rock climbing,” he says. “When I'm climbing, my mind clears up to solely focus on trying to find the most efficient way up a wall. Rock climbing is also a lovely way to spend time outdoors and it helps me refocus, especially when I’m facing a tough problem in the lab.”
Alongside his adviser Rob Patro, an associate professor of computer science with an appointment in the University of Maryland Institute for Advanced Computer Studies, Fan is working to build fast and modular algorithms for indexing huge collections of reference genomes.
Fan’s current goal is to design small data-structures—or indexes—to support fast sequence queries against tens of human scale genomes, or tens of thousands of bacterial genomes.
“Instead of requiring a large server or computer, my vision is to design tools that are so efficient they enable the same analysis on, say, powerful laptops,” Fan says.
He is also assisting on a $350K research grant recently awarded to Patro by the Chan Zuckerberg Initiative (CZI), a philanthropic research organization launched in 2015 by Meta founder Mark Zuckerberg and his wife, Priscilla Chan.
Patro, who leads the Combine Lab, is using the CZI funding to improve upon a “constellation” of interrelated tools his lab has developed to process genomic data. This includes alevin-fry, a toolkit for the efficient processing of single-cell and single-nucleus RNA sequencing data, and Salmon, a tool for efficient and accurate transcript quantification from bulk RNA sequencing data.
Fan is contributing by focusing on the development of Pufferfish2, which is set to improve the core index that powers Salmon and alevin-fry, as well as other sequencing tools that are currently in-the-works.
Fan, who grew up in Hong Kong, says he enjoys the interdisciplinary nature of his work the most. He received a prestigious National Science Foundation fellowship in 2020, which helps support his research.
“In the Combine Lab, we have to be good theorists and engineers to design and program efficient tools that work well,” Fan says. “Simultaneously, we must be able to exploit our understanding of biology to apply clever and effective heuristics (mental shortcuts) to make our tools work even better in practice. Almost always, these insights and exploits deriving from biological phenomena are what make our tools accurate and efficient.”
He says it is exciting to try to understand and explain why some complex computational and mathematical insights matter so much for biological analyses.
“It’s very cool and exhilarating to know that our work and the tools we build help other researchers investigate their own interesting biological questions,” Fan says.
—Story by Melissa Brachfeld, UMIACS communications group
Using Machine Learning to Classify Cancer Mutations

Growing up with professors for parents, junior and computer science major Ipsa Mittra was immersed in academia from the get-go. She knew from a young age that she wanted to conduct research to make a difference.
In high school, Mittra found her niche in computational biology through the STEM magnet program at Montgomery Blair High School in Silver Spring, Md. This spark of interest eventually led her to a prestigious summer fellowship this year conducting research at the Broad Institute and presenting at the 2022 National Diversity in STEM Conference.
“SACNAS was my first time presenting my research at a conference, and it was such a great experience,” Mittra said. “I got to meet so many other students like me conducting a range of interesting research, and it really solidified my plans to apply for graduate school as my next step.”
Mittra wasn’t the only Science Terp who presented at the Society for the Advancement of Chicanos/Hispanics and Native Americans in Science (SACNAS) National Diversity in STEM Conference in October. Physics graduate student Edgar Perez also presented his research in an oral presentation titled, “A Practical Source of Coherent Light from a High-Performance On-Chip Microresonator Optical Parametric Oscillator,” and postdoctoral astrophysicist Laura Vega gave an oral presentation on her research, “Simultaneous Multiwavelength Observations of the Highly Active M Dwarf YZ CMi.”
Presenting her research at SACNAS offered Mittra the opportunity to convey her Broad research to her peers, as well as faculty members from graduate programs she is interested in.
“I loved meeting other students at SACNAS who were presenting research that makes a difference,” Mittra said. “I also felt so special having graduate school faculty coming up to talk to me about my research; it was nice to see the applicability of the work and how other people that I look up to realize the stuff I’ve done is pretty cool.”
Finding Her Niche
In her high school computer science program, Mittra discovered that she wasn’t just interested in computer science—she was passionate about it.
“I did a summer research project in high school that was in the computational biology area, and that made me realize this is what I wanted to do in the future. That’s why I’ve been following that path,” Mittra said.
Mittra worked in Rameen Beroukhim’s lab at Broad, where she applied machine learning to examine structural variants of cancer mutations. To determine whether variants were germline (inherited) or somatic (developed in the body after conception), Beroukhim’s lab developed a computational approach.
The lab is using decades-old cancer sequence data sets—but because they were collected so long ago, they do not indicate what kind of mutation the cancer was (germline or somatic). To use these data sets to their full capacity, it’s important to identify whether the mutation was inherited or developed over time.
“Think of this classifier as the filter that sorts through what is a spam email and what belongs in your inbox. When you create an email account at first, you tell your email what is spam based on features like sender and message information,” Mittra said. “Eventually, the classifier picks up on the pattern itself and can make its own choices.”
Similarly, the classifier Mittra worked on examines data that has been pre-labeled as germline or somatic and identifies features that are important about structural variants so that that knowledge can be applied to classify unlabeled sequences. Understanding the roles of structural variants in cancer will help researchers develop more effective and tailored therapeutic responses for patients.
“During my summer fellowship, I got to go to the Dana-Farber Cancer Institute once a week to see the cancer patients being treated,” Mittra said. “It was a really big inspiration knowing the work I was doing had a direct application to someone that could potentially be getting treated for cancer.”
Pursuing Impact
Attending SACNAS gave Mittra the opportunity to be in a research-based environment surrounded by other people who are pursuing a career in academia—just like she is.
“I like computer science and I like what computer science can do, but I know that computational biology research is something that can help other people, and that’s really important to me,” Mittra said.
Mittra is currently working with UMIACS Director and Computer Science Professor Mihai Pop on gut microbiome research. Collecting RNA sequences from the gut microbiome, Mittra is testing approaches to clustering algorithms to separate the sequences into different bacterial organisms.
Soon, Mittra will apply to graduate programs specializing in computational biology. Meeting with graduate students and faculty members at SACNAS gave her clarity about the type of grad school community she wants to join and how she can contribute to making the academic community more inclusive once she becomes a professor.
“If you have a position of privilege as a researcher, you can empower other people by giving them those opportunities in research,” Mittra said. “You’re doing something small in one lab, but the work you’re doing could be crucial for the lab’s downstream to help cure an illness—and that’s so inspiring to me.”
—Story by Katie Bemb of the College of Computer, Mathematical, and Natural Sciences
Patro Awarded $350K to Improve Genomics Processing Tools

A University of Maryland expert on processing and organizing high-throughput genomics data has been awarded $350K from the Chan Zuckerberg Initiative (CZI), a philanthropic research organization launched in 2015 by Meta founder Mark Zuckerberg and his wife, Priscilla Chan.
Rob Patro (left in photo), an associate professor of computer science with an appointment in the University of Maryland Institute for Advanced Computer Studies, will use the CZI funding to improve upon a “constellation” of interrelated tools his lab has developed to process genomic data.
This includes alevin-fry, a toolkit for the efficient processing of single-cell and single-nucleus RNA sequencing data, and Salmon, a tool for efficient and accurate transcript quantification from bulk RNA sequencing data.
“A few of these tools have been broadly adopted within the genomics community,” Patro says. “This award will support our efforts to harden, modernize and expand the capabilities of these tools.”
By “harden,” Patro’s team aims to improve how the software is maintained and updated. They will accomplish this by creating suites of automated tests to ensure that new developments do not break existing tools in unexpected ways or introduce regressions.
In terms of modernizing, he says that a big part of the CZI project is converting certain tools from C++ to Rust, as the latter is a modern and “safe” programming language that makes developing and maintaining robust software much easier.
Finally, Patro says his team will add capabilities to these tools to process a wider range of data and handle different types of experiments.
“In addition to these goals, the grant also seeks to better document our core software components, both for users as well as for other developers, including scientists who can build off of our software,” he says. “Finally, there are diversity and outreach components, where we will seek to include in our developer community students and contributors from traditionally underrepresented groups.”
Patro, who is part of the Center for Bioinformatics and Computational Biology, says he appreciates the work CZI does as one of the few organizations that actively funds software.
“While most funding mechanisms support the development of tools as incidental artifacts arising from one’s research, they don’t generally provide support to build those tools out to an industrial scale, maintain or support them,” he says.
Assisting Patro on the project are Jamshed Khan, a fourth-year computer science doctoral student; Jason Fan, a fifth-year computer science doctoral student; and Dongze He, a fourth-year computational biology doctoral student. Noor Pratap Singh, a fourth-year computer science doctoral student will also assist in these efforts.
Patro’s winning proposal was one of 40 that were funded this year through CZI’s Essential Open-Source Software for Science program, which supports open-source software projects that are essential to biomedical research. Its goal is to support software maintenance, growth, development and community engagement for these critical tools.
—Story by Melissa Brachfeld
Using a Computational Approach to Improve Therapeutic Drug Treatments
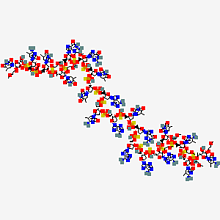
Caption: A molecular graphic inotersen, one of the antisense oligonucleotide drugs that will be studied to help improve therapeutic treatments for neuron damage and disease.
Michael Cummings, a professor of biology with an appointment in the University of Maryland Institute for Advanced Computer Studies, is part of a multi-institutional team that will apply powerful computational tools to make new drug therapies more effective.
The two-year project is funded by a $300K grant from the U.S. Food and Drug Administration.
The team, which includes faculty members from the University of Maryland School of Pharmacy and the University of Maryland Institute for Bioscience and Biotechnology Research (IBBR), will analyze the synthetic process that goes into manufacturing antisense oligonucleotides—small pieces of DNA or RNA that can bind to specific molecules of RNA.
These antisense agents can be used in the treatment of various diseases, including neurological disorders, cancer, cardiovascular and metabolic diseases.
Cummings, who is director of the Center for Bioinformatics and Computational Biology, says the synthesis process used to make these drugs results in products of different chemical configurations, termed diastereomers. Existing research has shown that different diastereomer compositions may alter their pharmacological effects, potentially rendering them not as beneficial.
To better understand how the synthesis process may affect the diastereomeric compositions in the drug product, the FDA-funded team will employ a multidimensional analytical approach that computationally integrates state-of-the-art liquid chromatography, mass spectrometry and nuclear magnetic resonance spectroscopy.
Cummings says their research will focus on two specific drugs. One is used to treat children and adults with spinal muscular atrophy, an inherited motor neuron disease that causes skeletal muscle weakness and wasting, which worsens with age. The other is used to treat adults with hereditary transthyretin-mediated amyloidosis, which results in damage of multiple nerves throughout the body.
Cummings will analyze the laboratory results and construct models of the drug synthesis process to better predict diastereomer compositions.
Assisting on the project are Jace Jones, an assistant professor of pharmaceutical sciences and associate director of the Mass Spectrometry Center at the University of Maryland School of Pharmacy; Steven Fletcher, an associate professor of pharmaceutical sciences; and Robert Brinson, a research chemist at IBBR and the National Institute of Science and Technology.
—Story by Melissa Brachfeld
